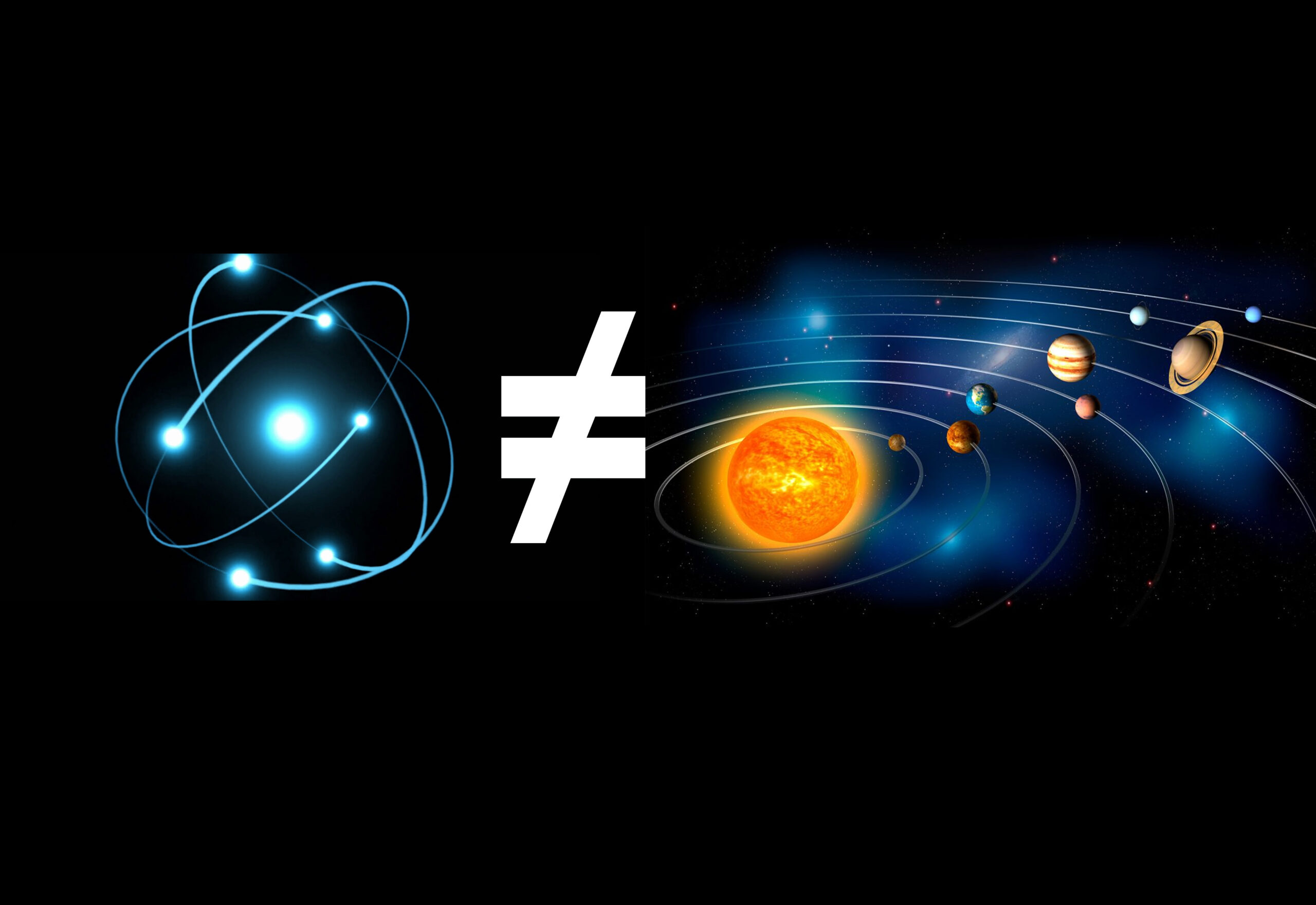
Atoms have long been a subject of fascination for scientists and laypersons interested in natural sciences. At the dawn of the atomic era, many physicists and chemists used the metaphor of a miniature solar system to explain the structure of the atom. However, as our understanding of physics and chemistry has advanced, we now know that atoms are not, in fact, miniature solar systems.
The very large compared with the very small
This article will explore why comparing atoms to solar systems is a bad example and why updating our educational models to reflect current knowledge is essential. Let us first define what an atom and a planet is, respectively:
An atom is the smallest unit of matter that retains the chemical properties of an element. Atoms comprise three types of particles: protons, neutrons, and electrons. Protons have a positive charge, electrons have a negative charge, and neutrons are neutral. Protons and neutrons are found in the nucleus, located at the center of the atom, while electrons orbit the nucleus in shells or energy levels.
The discovery of the atom began in the late 1800s when scientists observed that matter could not be divided indefinitely and that all matter consisted of tiny, indivisible particles. In 1897, J.J. Thomson discovered the electron, a negatively charged particle that orbits the nucleus of an atom. In 1911, Ernest Rutherford conducted the famous gold foil experiment, which revealed that the atom had a small, dense nucleus that contained positively charged particles called protons. In 1932, James Chadwick discovered the neutron, a neutral particle in the nucleus.
The number of protons in an atom determines its atomic number and identifies the element to which it belongs. For example, an atom with six protons is carbon, while an atom with eight is oxygen. The number of neutrons can vary, resulting in different isotopes of the same element with slightly different masses. The electrons in an atom are arranged in shells, with the first shell holding up to two electrons and the second shell holding up to eight electrons.
Atoms are tiny and cannot be seen by the human eye, with diameters ranging from 0.1 to 0.5 nanometers. They make up all detectable matter in the universe.
On the other hand, a planet is a celestial body that orbits around a star, is large enough to be rounded by its gravity, and has cleared its orbit of other debris. Planets are commonly composed of rock and gas and are found in various sizes and distances from their star. They are characterized by their physical properties, such as their mass, radius, density, and surface features, as well as their orbital characteristics, such as their distance from their star, their period of revolution, and their eccentricity. Planets play a crucial role in the study of astronomy and planetary science, as they provide insights into the formation and evolution of our solar system and the broader universe.
The discovery of planets in our solar system began in ancient times, with the naked-eye observations of the five visible planets: Mercury, Venus, Mars, Jupiter, and Saturn. The discovery of Uranus in 1781 and Neptune in 1846 marked significant milestones in astronomy, as they were the first planets discovered using telescopes. Pluto, initially considered the ninth planet, was discovered in 1930 by Clyde Tombaugh using photographic plates. However, in 2006, Pluto was reclassified as a “dwarf planet” due to its small size and different characteristics from the other planets in our solar system. The discovery of other planets in our solar system has expanded our knowledge of our cosmic neighborhood and the variety of planetary systems.
Pedagogical problem
Now, one of the main issues with the metaphor of the miniature solar system is that particles behave very differently on a small scale than on a large scale. The laws of classical physics, which govern the behavior of macroscopic objects, break down at the atomic level. Instead, we must rely on the principles of quantum mechanics to describe the behavior of particles at this scale. Unlike the predictable motion of planets in a solar system, the motion of particles at the atomic level is governed by probability waves. The position of an electron in an atom cannot be precisely determined but is instead described by a probability cloud. This intrinsic feature of the universe means that the electron does not orbit the nucleus like a planet orbits a sun but exists in a probability state around the nucleus.
The miniature solar system metaphor could be better for education because it perpetuates an outdated understanding of the atom. This model was developed in the early 20th century, before the development of quantum mechanics. While it was helpful at the time for conceptualizing the atom, it is no longer an accurate representation of its structure. Continuing to teach this model in schools and universities can lead to confusion and misconceptions about the true nature of atoms.
Correspondingly, the metaphor of the miniature solar system is problematic because it implies that the behavior of atoms can be fully understood through classical physics. In reality, the behavior of particles at the atomic level is governed by the principles of wave and particle behavior, known as wave-particle duality. Depending on the circumstances, particles can exhibit wave-like and particle-like behavior, making the behavior of atoms more complex than that of a solar system.
The combination of general relativity and quantum mechanics has been a long-standing challenge in theoretical physics. Both theories describe the universe’s behavior at different scales and under different conditions but use fundamentally different mathematical frameworks and assumptions. General relativity describes the behavior of gravity on a macroscopic scale, while quantum mechanics describes the behavior of particles on a microscopic scale. The biggest challenge in combining these two theories is that they make contradictory predictions in extreme conditions such as black holes or the Big Bang. This peculiarity has led to several proposed theories, such as string theory and quantum loop gravity, but a consistent framework is still elusive.
Teach good
The comparison between atoms and miniature solar systems is a bad example. Not only does it perpetuate an outdated understanding of the atom’s structure, but it also implies that the behavior of particles at the atomic level can be fully understood through classical physics. This circumstance is not the case, as quantum mechanics and wave-particle duality govern the behavior of particles. As our understanding of physics and chemistry evolves, we must update our educational models to reflect these discoveries. By doing so, we can ensure that the next generation of scientists clearly and accurately understands the world around us.
Recommended articles
★ ★ ★ ★ ★
This is an original article published exclusively by Space Expert. You may cite it as:
"The atom is not a miniature solar system" in Space Expert, 2026
Permalink: https://space-expert/the-atom-is-not-a-miniature-solar-system/



Заказать диплом института по выгодной цене возможно, обращаясь к надежной специализированной фирме. Для проверки компании до покупки воспользуйтесь рейтингом и отзывами в онлайне. Так можно приобрести диплом из любого института России ralphouensanga.com/read-blog/62761_kupit-diplom-o-vysshem-obrazovanii.html
“VN88 là nền tảng giải trí trực tuyến được xây dựng theo hướng ổn định, dễ sử dụng và thân thiện với người dùng.
Website : http://vn88be.com/
VN88“